Production of human vaccine
Based on GMP standards and WHO requirements, the human vaccine production line of the knowledge-based company, Bayerpl Pharmatech, operates in a cleanroom facility spanning over 3000 square meters, classified as C, B, A. Equipped with advanced machinery, the production capacity exceeds 20 million doses of various human vaccines.
Currently, the company has introduced seasonal trivalent and quadrivalent influenza vaccines as its first products, with plans to launch vaccines for measles, mumps, and rubella soon.
The research stages for the production of injectable polio vaccine and rotavirus vaccine are underway at this company.
| ®Flopak | Seasonal Influenza vaccine (Trivalent) |
| ®Flopak | Seasonal Influenza vaccine (Quadrivalent) |
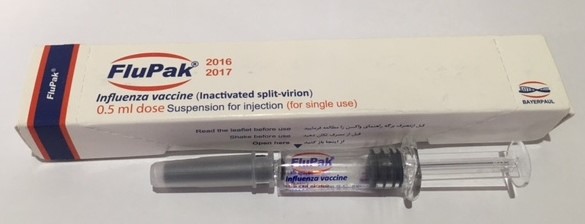
Seasonal Trivalent Influenza Vaccine Flopak®
The first vaccine produced on the production line of Bayerpl PharmaTech is the influenza vaccine (FluPak). The vaccine is manufactured in compliance with GMP standards, WHO requirements, and regulations set by the Ministry of Health’s Food and Drug Administration. The FluPak vaccine is produced by cultivating the virus on eggs and formulated without the use of thiomersal preservatives. It is available in pre-filled syringes in a volume of half a milliliter since 2018.
Seasonal Quadrivalent Influenza Vaacine Flopak®
The vaccine is manufactured in compliance with GMP standards, WHO requirements, and regulations set by the Ministry of Health’s Food and Drug Administration. The FluPak vaccine is produced by cultivating the virus on eggs and formulated without the use of thiomersal preservatives. It is available in pre-filled syringes in a volume of half a milliliter since 2018.

